Is Cf3coo- an Acid or Base
Answer 1 of 2. View the full answer.
Trifluroacetate C2f3o2 Chemspider
A towards the products because Q Kc B towards the products because Q Kc C towards the reactants because Q Kc D towards the reactants because Q Kc 21.
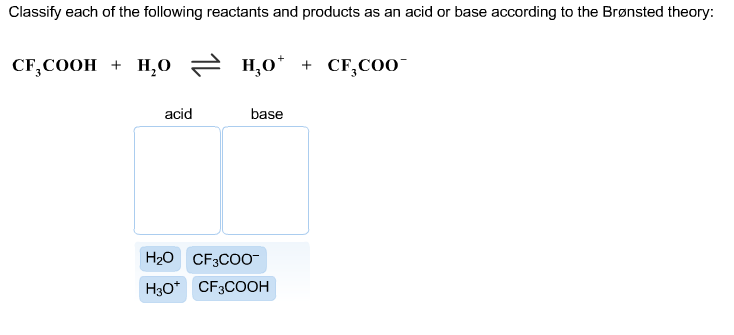
. Consider the acid-base reaction and classify each of the reactants and products as an acid or base according to the Brønsted theory. Try drawing the structure of the conjugate base ceCF_3COO-. CF3COOH H20 근 H30 CF3COO- Acid Base Answer Bank CECoO CFCOOH 8 9 6 This problem has been solved.
According to the Bronsted-Lowry acid base theory a base is. So CH 3 COOH is a weak acid that forms a conjugate base according to the concept of conjugate acid-base pair. A anything that gives up OH- in solution.
I am having a difficult time understanding what makes a buffer a bufferBuffers in my textbook are defined as a solution of a weak acid or base and their conjugate acidbase. Acetic acid also named named ethanoic acid is an organic acid with the chemical formula. Its a weak base because its conjugate acid is weak.
For example Vinegar is an acid and measures 24 on the pH scale. The ion CH3COO- acetate ion is mildly basic. It is made out of Carbon Hydrogen and Oxygen.
100 1 rating ACF3COOHH2OH3OCF3COO- Acid1 base2 acid2 base1 B. Acetic acid incompletely dissociates into an acetate ion the conjugate base and a Hydrogen ion. Weak Very weak.
The pairings are Acid. NaC2H3O2 is a basic salt because the acetate ion will pull an H ion from water to form the weak acid CH3COOH Acetic acidSince acetic acid is weak it will mostly remain in water as is which will leave some OH- ions floating around. CFCOOH H0 H0 CF COO Base Acid Answer Bank HO CF000 10 CHCOOH.
Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added Figure 1414. Consider the acidbase reaction and classify each of the reactants and products as an acid or base according to the brønsted theory. A solution of acetic acid and sodium acetate CH3COOH CH3COONa is an example of a buffer that consists of a weak acid and its salt.
So if I were to just dissolve acetic acid a weak acid in water why would this not be defined as a buffer. Click to see full answer Also is NaC2H3O2 acidic basic or neutral. The conjugate base of CH3COOH is Acetate CH 3 COO-.
The scale ranges from 0-14. Helpful 1 Not Helpful 1 Add a Comment. Ch3coo- is base What is an acid base neutral.
B a proton donor C a proton acceptor D an electron pair acceptor. So for a stronger acid lower cepK_a the negative charge must be more stabilized. In general the more stabilized the negative charge of the conjugate base is the more the equilibrium favors that form thus the more the acid dissociates thus the stronger the acid is.
CH3COOH is Acetic Acid. Keeping it similar to the general acid properties Arrhenius acid also neutralizes bases and turns litmus paper into. It is a structural analogue of acetic acid with all three of the acetyl groups hydrogen atoms replaced by fluorine atoms and is a colorless liquid with a vinegar.
Maxwell presented by Consider the acid-base reaction and classify each of the reactants and products as an acid or base according to the Brønsted theory. The colour of the paper matches up with the numbers on the pH scale to indicate what kind of substance is being tested. Why CH3COOH is a monoprotic acid.
Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. Trifluoroacetic acid TFA is an organofluorine compound with the chemical formula CF 3 CO 2 H. Litmus paper is an indicator used to tell if a substance is an acid or a base.
Very weak means it does not act as a base or acid when you dissolve it in water. Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions. Consider the acid-base reaction and classify each of the reactants and products as an acid or base according to the Brønsted theory CF3COOH H20 H30 CF3C00 CF3COOH.
The OH- ions floating around makes the solution basic. CF3COOH H20 근 H30 CF3COO- Acid Base Answer Bank CECoO CFCOOH 8 9 6 Question.
Trifluoroacetic Acid Cf3cooh Pubchem
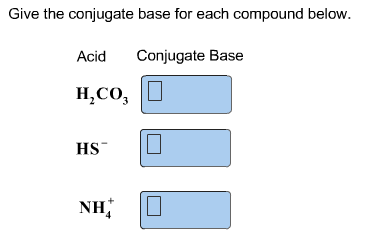
Solved Classify Each Of The Following Reactants And Products Chegg Com

Solved A What Is The Conjugate Acid Of Cf3coo Enter Chegg Com

Solved Classify Each Of The Following Reactants And Products Chegg Com
Comments
Post a Comment